
Yousang Gwack, PhD
Position Title:Associate Professor, Physiology
Biography:
Yousang Gwack, PhD received his B.S., M.S., and PhD degrees from Korea Advanced Institute of Science and Technology (KAIST). His Masters and PhD work at Dr. Joonho Choe’s lab focused on the mechanisms underlying viral (human papillomavirus, hepatitis C virus and herpesvirus) invasion into the host immune system. As a postdoctoral researcher in Harvard Medical School, he continued investigating the mechanism by which viral transcription factors influence host cell transcription events under the guidance of Dr. Jae U Jung (currently Chair, Department of Molecular Microbiology and Immunology, USC). Later, Dr. Gwack changed his research field to molecular immunology to investigate one of the fundamental signaling pathways for T cell activation, the Ca2+-NFAT pathway in Dr. Anjana Rao’s lab (Harvard Medical School, currently, La Jolla Institute for Allergy and Immunology). He designed and carried out two genome-wide RNAi screens that led to identification of novel regulators of the NFAT family of transcription factors, a dual specificity kinase, DYRK and the pore subunit of the CRAC channel Orai1. After moving to UCLA as an assistant professor in 2007, Dr. Gwack expanded his research to identify novel regulators for the Ca2+-NFAT and other signaling pathways using molecular biology approaches, and to investigate the physiological role of this pathway in effector T cells and other immune cells using knockout mice.
Research Interests:
Ca2+ signaling is critical for the function of immune cells. Previously, our group identified Orai1 as the long-sought pore component of the predominant Ca2+ channels in immune cells. In collaboration, we identified patients with a genetic mutation in Orai1 that causes lethal, severe combined immune deficiency. Considering the crucial role of Orai1 in the immune system, understanding of its regulatory mechanism is crucial for development of new therapies targeting diseases caused by hypersensitive immune reaction such as type I diabetes, rheumatoid arthritis, multiple sclerosis, and psoriasis. The main goals of our research are; (i) basic studies to understand the regulatory mechanism of the Orai1-NFAT pathway, (ii) development of animal model systems to test the therapeutic potential of blocking Orai1, and (iii) identification of methods to modulate Orai1 activity to balance the immune system. The developing research areas of interest include; (i) broad understanding of the role of the Orai1-NFAT pathway in various cell types (e.g., stem cells, excitable cells, and innate immune cells), and (ii) elucidation of crosstalk between the Orai1-NFAT pathway and other signaling pathways. These goals will be achieved by various tools including proteomics (large-scale protein purification and mass spec analysis), genome-scale or chemical library high throughput screens, advanced microscopy in live cells, transcriptome analyses, and animal disease models of infection and autoimmunity.
Awards/Honors:
2013 Outstanding Tutor Award, Problem-Based Learning
2012 Outstanding Tutor Award, Problem-Based Learning
2012 Research Grant Award, Lupus Research Institute
2011 Outstanding Tutor Award, Problem-Based Learning
2010 Outstanding Tutor Award, Problem-Based Learning
2009 Cancer Research Coordinating Committee Award
2009 Research Services Research Fund (RSRF)
2008 Stein Oppenheimer Endowment Award
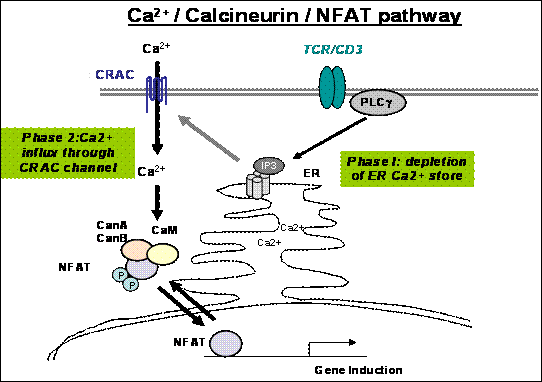
Schematic diagram showing two phases of activation of store-operated Ca2+ entry in T cells. The ER Ca2+ store is depleted by IP3 produced by PLC-gamma activated through the ligand-receptor engagement (Phase 1). The depletion can trigger opening of the CRAC channels on the plasma membrane (Phase 2). The long sought genes encoding the CRAC channel component was found by genome-wide RNAi screen (Nature, 441, p179) and named as “Orai”.